Answer
The number of atoms in 0.07 moles of lithium is 4.2161 x 10²² atoms.
Step-by-step explanation
Given:
Moles of lithium = 0.07 mol
What to find:
The number of atoms in 0.07 moles of lithium
Step-by-step solution:
From Avogadro,
1 mole of a substance contains 6.023 x 10²³ atoms.
So 0.07 mole of lithium will contains
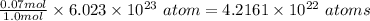
The number of atoms in 0.07 moles of lithium is 4.2161 x 10²² atoms.