We will start by determining the new concentration of the HCl solution. To do this we will calculate the dilution factor then use this dilution factor to calculate the new concentration:
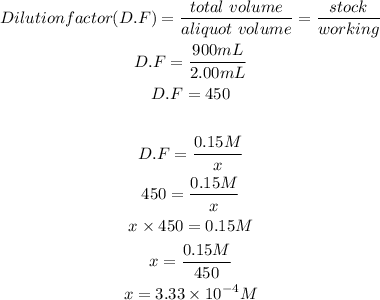
Now we will calculate the pH,
![\begin{gathered} pH=-log[H^+] \\ pH=-log[3.33*10^(-4)] \\ pH=3.48 \end{gathered}](https://img.qammunity.org/2023/formulas/chemistry/college/f01bv1vqgrssoj8c3zay6jnn6fwvqr7med.png)
Answer: The pH of the diluted solution 3.48,