Since no sugar will be added or removed, the number or moles of it in the glas won't change.
Let's call this quantity:
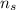
The molar concetrnation can be written as the followin equation:
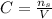
Where C is the concentration given the number of moles of sugar and the volume V.
We can rewrite this as:
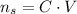
Now, before the ice melts, the volume of the lemonate is 150 mL and the sugar concentration is 2.27 mol/L. Let's call this situation 1:
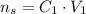
After the ice melt the one third it will, we will have a certain volume and the concentration we want 1.88 mol/L. Let's call this situation 2:
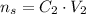
Now, we can put thouse tofether:
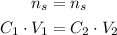
And we can solve for the unknown volume of situation 2:
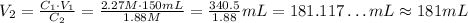
Since the final volume is approximately 181 mL, the difference between it and the initial volume is the volume of water that came from the melted part of the ice:
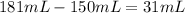
Since we assume that the density of the water is 1.00 g/mL, we can calculate the mass it represents:
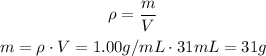
And since this is only 1 third of the ice (the rest won;t melt), we know that the whole ice will have three times this mass:
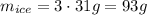
So, it should be added approximatelt 93 grams of ice.