Answer:
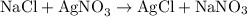
Explanations:
What are balanced equations?
A chemical reaction is known to be balanced if the total number of moles at the reactant is equal to that of the product.
Recall that double decomposition reaction is the reaction whose result is the interchange of two parts of two substances to form two new substances.
According to the question, we are to write the balanced chemical reaction between sodium chloride (NaCl) and silver nitrate (AgNO₃).
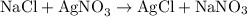
As you can see from the reaction, chlorine combines with silver to form silver chloride at the product side, and sodium combines with nitrate (NO₃) to form sodium nitrate.