The formula of molarity is the following:
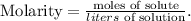
But, we need to calculate the number of moles in 32.5 g of HBr using its molar mass which is 80.9 g/mol. The conversion would be:
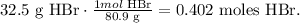
Now that we have the number of moles, we can solve for 'liters of solution' from the formula and replace the values of molarity (0.500 M) and moles (0.402 moles):

The answer is that the volume required for a 0.500 M of 32.5 g of HBr is 0.804 L.