INFORMATION:
We know that:
- A vessel contains nitrogen gas and has a total mass of 7.0 g
- The temperature is 400K
- The pressure is 12.0 atm
And we need to find the density
STEP BY STEP EXPLANATION:
To find the density, we need to know that
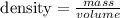
We know that the mass is 7.0 g, but we don't know the volume. So, we need to calculate the volume.
To calculate the volume we need to use that it has an ideal gas behavior. That means that we can use the formula for ideal gas

Where,
- P is the pressure
- V is the volume
- n represents the moles of the gas
- R is the universal constant
- T is the temperature
In the statement is given that,
- P = 12.0 atm
- V = ? (we must find it)
- n = 0.2498 mol
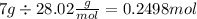
- R = 0.08206 (atm * L) / (mol * K)
- T = 400 K
Now, replacing in the formula
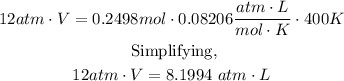
Then, solving for V
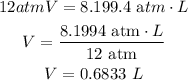
Finally, knowing that the mass is 7.0 g and the volume is 0.6833 L, the density will be
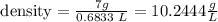
ANSWER:
The density is 10.2444 g/L