INFORMATION:
We know that:
- Mia has a 32.0 g sample of Fe(OH)3 for the investigation purpose.
And we must find how many grams of oxygen are recovered from the sample
STEP BY STEP EXPLANATION:
To find the amount of gram of oxygen, we must:
1. Calculate the percent of oxygen in compound
- Find the molar mass of the compound
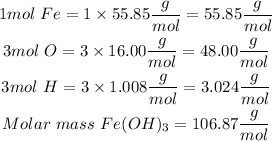
- To find the percent by mass, divide the part by the whole and multiplying by 100.
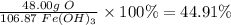
2. Multiply the starting mass by the percent of the desired element.
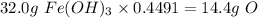
Finally, 14.4 g of oxygen are recovered from the sample.
ANSWER:
14.4 g