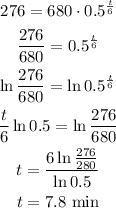Given
half life in 6m
Initial 680g
Procedure
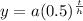
This is the model we can use to calculate the decay. But we must recognize the values of a and of h
The value of a corresponds to the initial value of the population, which in our case is 680g.
While the value of h will be the number of minutes it takes to reach its half-life. Therefore:
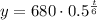
With this formula, we can now proceed: