ANSWER
The mass of oxygen is 12.279 grams
Explanation:
From the question provided, you were given the volume of oxygen to be 17.2 liters.
Given information
The volume of oxygen = 17.2 liters
To find the mass of oxygen, we need to find the mole of oxygen first
Recall that, at S.T.P, 1 mole is equivalent 22.4L
Let the mole of oxygen be x
The next step is to find the mole of oxygen using the above relationship
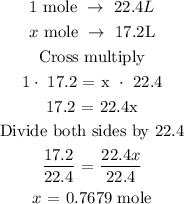
The mole of oxygen is 0.7679 mole
From the periodic table, the molar mass of oxygen is 15.99 g/mol
The next thing is to find the mass of oxygen using the mole formula

Therefore, the mass of oxygen is 12.279 grams