They give us the balanced equation of the reaction for the production of ammonia. We see that for every mole of N2 that reacts, two moles of ammonia are formed. This corresponds to the ratio, that is, the ratio N2 to NH3 is 1/2. therefore, if 0.40 mol of MH3 is obtained, the moles of N2 that reacted were:
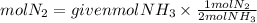
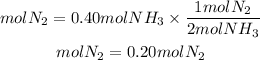
Answer: It reacted 0.20 mol of N2