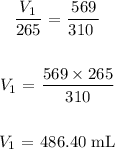Answer:
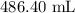
Step-by-step explanation:
Here, we want to get the initial volume of air
From Charles' law, we know thattemperature and presure are directly proportional at consnstant pressure
mathematically:
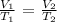
Where:
V1 is the original volume which is ?
V2 is the final vlume which is 569 mL
T1 is the initial temperature which we would convert to absolute scale by adding 273 K : -8 + 273 = 265 K
T2 is 37 deg Celsius which is 273 + 37 = 310 K
Substituting the values: