In order to determine the heat capacity of the liquid, use the following formula for the heat gained by the liquid:
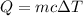
where
m: mass = 13.0 g
c = ?
ΔT: change in temperature = 1.73 ∘C
Q: heat gained by the liquid = 47.3 J
solve the equation for c and replace the previous values:
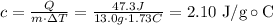
Hence, the heat capacity of the liquid is 2.10 J/g∘C