We need to use that
In order to clasify the salt we need to use that:
- A neutral solution is formed when a strong acid reacts with a strong base
- An acidic solution is formed when a strong acid reacts with a weak base
- A basic solution is formed when a weak acid reacts with a strong base.
In this case, we have a weak acid and a strong base. Then, the solution is a basic solution.
Explaining it in terms of hydrolysis,
When dissolving HCOOK in water, as it is a strong electrolyte, it will be completely dissociated:
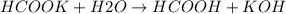
- the strong base kOH, has very little acid character so it will not react with water
- HCOOH, which being the conjugate base of a weak acid, H2COOH, will be strong enough to capture protons to water, which will behave like an acid, undergoing the hydrolysis reaction:
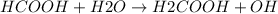
The presence of OH ions in the aqueous solution gives it a certain basic character, which will depend on the degree of intensity with which the previous hydrolysis equilibrium is shifted "to the right".
ANSWER:
The solution is a basic solution.