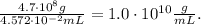Remember that for multiplication and division problems, the answer should be recorded to the same number of significant figures as the measurement with the least number of significant figures.
In this case, the mass has 2 significant figures but volume contains 4 significant figures, so the answer needs to have 2 significant figures: