Answer:
Explanations:
First we need to determine the moles of base using the formula;
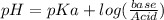
where:
• pKa of Na3PO4 = 1.8
,
• pH =7.75
Determine the mole of the acid
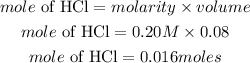
Substitute the given parameters
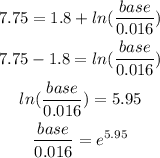
From the calculation above, the base is calculated as
Base = 0.016e^5.95
Base = 6.14 moles
Determine the mass of the compound
![undefined]()