Answer:
B. Yes, and there will be extra left over.
Step-by-step explanation:
1st) It is necessary to balance the chemical reaction:
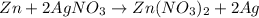
Now we know that 1 mole of zinc (Zn) reacts with 2 moles of silver nitrate (AgNO3) to produce 2 moles of pure silver (Ag).
We can convert the grams of each compound, using the molar mass of Zn (65.4g/mol), AgNO3 (170g/mol) and Ag (108g/mol), and we can see that 65.4g of Zn react with 340g of AgNO3 to produce 216g of Ag.
2nd) Using the given value of pure silver (800g) and the stoichiometry of the reaction, we can calculate the amount of Zn and the amount of AgNO3 that will be needed:
• Zn:
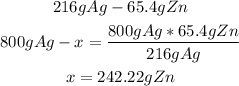
• AgNO3:
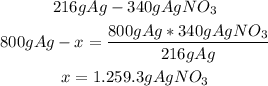
Finally, to produce 800g of Ag they will need 242.22g of Zn and 1,259.3g of AgNO3, so they be able to make enough silver to fill the order and there will be extra left over.