Answer
48.3 °C
Step-by-step explanation
Given parameters:
Mass of the chunk material, m = 328.0 g
The specific heat of the material, c = 1.17 J/g°C
Initial temperature, T₁ = 71.0 °C
The heat lost, Q = 8.7 kJ = 8700 J
What to find:
The final temperature (T₂) of the chunk after it loses 8.7 kJ of heat in a pot of water.
Step-by-step solution:
The final temperature (T₂) of the chunk after it loses 8.7 kJ of heat in a pot of water can be calculated using the formula for heat lost.
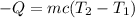
Putting the values of the given parameters into the formula, we have:

Therefore, the final temperature of the chunk after it loses 8.7 kJ of heat in a pot of water is 48.3 °C.