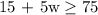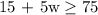
Firstly, let the number of weeks be w
If she saves $5 in a week
Then in w weeks, the amount saved will be 5 * w = $5w
If we add this to the extra $15, the total becomes 15 + 5w
Now, let us look at her goal
The goal is that the savings must be at least $75
What this mean is that, it must be 75 or more, but it cannot be less
The correct inequaity will be;