To find the answer to this question, we have to use Avogadro's number, which is the number of molecules present in one mole of any substance.
It states that there are 6.022x10^23 molecules of oxygen in one mole of oxygen.
Use this number to find the number of moles in 2.84x10^95 molecules of oxygen:
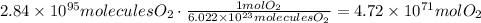
There are 4.72x10^71 moles of oxygen.