ANSWER
The mass of Cl2 gas is 88.75 grams
Step-by-step explanation
Given that;
The mass of NaCl is 146.1 grams
Follow the steps below to find mass of chlorine gas
Step 1; Write the balanced equation of the reaction
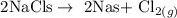
Step 2; Find the number of moles of chlorine gas using the below formula
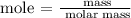
Recall, that the molar mass of NaCl is 58.44 g/mol
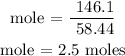
The number of moles of NaCl is 2.5 moles
Step 3; Find the number of moles of Cl2 using a stoichiometry ratio
Let x represents the number of moles of chlorine
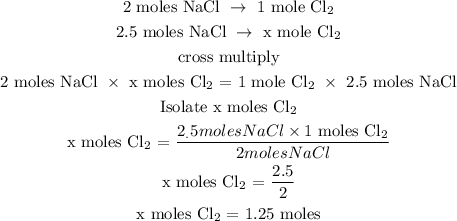
The moles of Cl2 is 1.25 moles
Step 4; Find the mass of Cl2
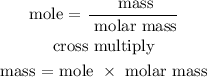
molar mass of Cl2 is 71 g/mol
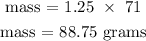
The mass of Cl2 gas is 88.75 grams