To find the enthalpy of the reaction we can use the bond energies of the compounds present in the reaction. First we need to make sure that the reaction equation is balanced, and we are effectively given the balanced equation since we have two hydrogens and two chlorines on each side of the reaction.
Now, the enthalpy of reaction will be equal to:
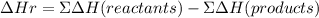
The sum of the formation energy of the reactants, dH(reactants) will be:
dHf, corresponds to the bond energy given in the table.
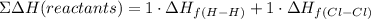
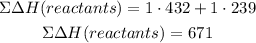
The sum of the formation of the products will be:
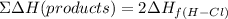
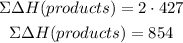
The energy of the reaction will be:

The reaction is exothermic as we obtain a negative value of the energy of the reaction.
In summary, to obtain the reaction energy we must:
• Sum the bond energies of the reactants.
,
• Sum the bond energies of the products.
,
• Make the difference of the sums of bonds energy of the reactants minus the products, if the difference is negative the reaction will be exothermic, otherwise, the reaction will be endothermic. In this case, we have a negative value equal to -183.