Answer: the pressure exerted by the gas is 652 x 10^3 Pa, which corresponds to 652 kPa
Step-by-step explanation:
The question requires us to calculate the pressure, in kPa, connsidering the following information:
number of moles = n = 4.20mol
volume of gas = V = 15.0L
temperature of gas = T = 280.0 K
We can use the equation of ideal gases to calculate the pressure of the gas, as shown by the rearranged equation below:
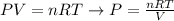
Since the volume was given in L and the question requires us to calculate the pressure in kPa, we can use R in units of L.Pa/K.mol:
R = 8314.46 L.Pa/K.mol
Applying the values given by the question to the rearranged equation above, we'll have:
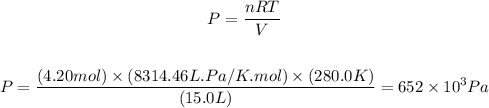
Therefore, the pressure exerted by the gas is 652 x 10^3 Pa, which corresponds to 652 kPa.