In order to calculate the final temperature, we can use the formula below:
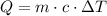
Where Q is the heat energy (in Joules), m is the mass, c is the specific heat and Delta T is the change in temperature (final temperature minus initial temperature).
So, using Q = 10312 J, m = 4 kg, c = 390 J/kg°C and Ti = 28°, we have:
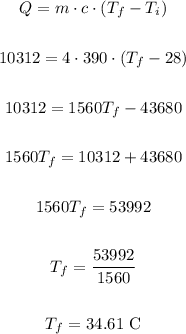
Therefore the final temperature is 34.61 °C.