Given data
*The given mass of mercury is m = 10.0 kg
*The value of latent heat of vaporization of mercury is
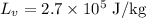
The formula for the heat is needed to vaporize 10.0 kg of mercury at its boiling point is given as
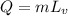
Substitute the known values in the above expression as
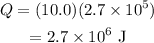
Hence, the heat is needed to vaporize 10.0 kg of mercury at its boiling point is Q = 2.7 × 10^6 J