Answer:
13%.
Step-by-step explanation:
What is given?
Molar mass of C = 12 g/mol.
Molar mass of H = 1 g/mol.
Molar mass of N = 14 g/mol.
Molar mass of Na = 23 g/mol.
Molar mass of O = 16 g/mol.
Molar mass of S = 32 g/mol.
Step-by-step solution:
First, we have to calculate the molar mass of C14H14N3NaO3S by doing an algebraic sum with the given molar masses of each element. We have 14 carbons (C), 14 hydrogens (H), 3 nitrogens (N), 1 sodium (Na), 3 oxygens (O), and 1 sulfur (S). The calculation of this molar mass will look like this:
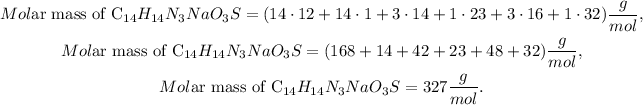
Now that we have the molar mass of methyl orange (C14H14N3NaO3S), we have to calculate the composition of nitrogen. As we have 3 nitrogens and the molar mass of nitrogen is 14 g/mol, the molar mass of this only element in methyl orange would be 3 x 14 = 42 g/mol, so the composition formula is given by this:
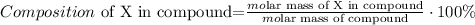
X would be N (nitrogen) and the compound would be C14H14N3NaO3S, so replacing the values that we have, we obtain:
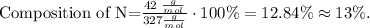
The composition of nitrogen in methyl orange would be 13%.