Step 1 - How specific heat relates to heat
The heat absorbed by a substance can be related to its mass (m) and temperature (delta T) variation by the following formula:
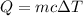
We will use the following formula to solve the poblem.
Step 2 - Solving the exercise
According to this exercise:
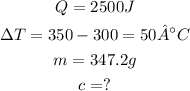
Setting the values in the equation:
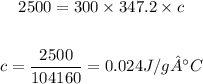
Answer: the specific heat of this metal is 0.024 J/g.°C