Answer:
46.64 hours
Explanation:
The amount, A(t) of radioactive material with a half-life of t(0.5) remaining after time t is modeled using the formula below:
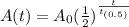
• A(t)=quantity of the substance remaining
,
• A(0)=initial quantity of the substance
,
• t=time elapsed
,
• t(0.5)=half life of the substance
In our case:
• A(t)=350 mg
,
• A(0)=500 mg
,
• t = 24 hours
Substitute into the formula above to find the half-life, t(0.5).
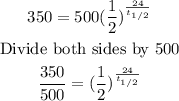
Take the natural logarithm of both sides.
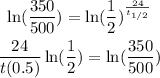
Divide both sides by ln(1/2).
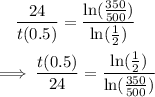
Multiply both sides of the equation by 24.
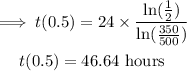
The half-life of the radioactive element is approximately 46.64 hours.