So,
The balanced reaction for this situation is:
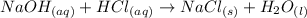
The first thing we're going to do is to determine the # of moles of HCl from its volume and molarity: (Remember that 5mL are 0.005 liters)
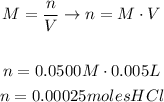
Now, we're going to convert these moles to moles of NaOH using stoichiometry:
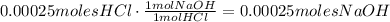
And then, use the calculated moles of NaOH and its molarity (0.100 M) to figure out the volume needed.
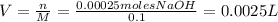
If we pass this answer to mL:
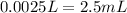
Therefore, we need 2.5mL of NaOH to neutralize the solution.