So we have to find the equation of a line that passes through (2,4) and has a slope of 1/2 in standard form. First, we should write it in point-slope form because it will be easier to find it. Then we can change it into the standard form. The point-slope form of a line with a slope m that passes through point (a,b) is:
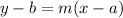
In this case we are told that the slope is 1/2 and the point is (2,4) so we get:
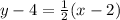
Now that we have the equation in point-slope form we must convert it into the standard form. This form looks like the following:
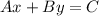
Where A, B and C are numbers. So let's take the equation we found before and distribute the product in the right side:
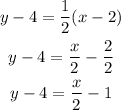
Now let's add 4 to both sides:
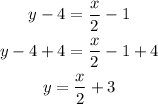
We substract x/2 from both sides:
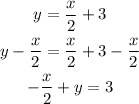
And finally we multiply both sides by 2:
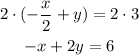
Then the answer is option b.