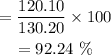Answer
Percent Carbon in C10H10 = 92.24 %
Step-by-step explanation
To know the percent carbon by mass in C10H10, We need to first calculate the molecular mass of C10H10
The molar mass of Carbon, C = 12.01
The molar mass of Hydrogen, H = 1.01
The molecular mass of C10H10 =
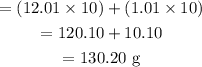
The total number Carbon in the compound is 10
Therefore, mass in gram of carbon in the compound = 12.01 x 10 = 120. 10 g
Percent Carbon in C10H10 =