To relate the change in temperature of a mass of certain material with the given heat, we can use the following equation:
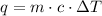
Where q is the heat, m is the mass, c the heat capacity of the material and ΔT is the temperature variation.
So, we can solve for m and substitute the kown values:
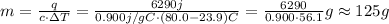
So, the mas of aluminium is approximately 125g.