The ratio of the area of triangles ABC to the area of triangle XYZ is 1/9
STEP - BY - STEP EXPLANATION
What to find?
The ratio of the area of triangles ABC to the area of triangle XYZ
To solve the given question, we will follow the steps below:
Step 1
Find the value of the base of triangle XYZ (length XY).
From the given figure, YW = WX
⇒YW = WX =15
⇒ XY = WX + YW = 15 + 15 = 30
Hence, the base = 30.
Step 2
Identify the value of the height of the triangle XYZ.
Height =36
Step 3
Recall the formula for calculating the area of a triangle.
Area = base x height
Step 4
Find the area of triangle XYZ.
Substitute the value of the base and height into the formula in step 3 and simplify.
That is;
Area = 30 x 36 =1080
Step 5
Determine the height of triangle ABC (length CD).
Using the trigonometric ratio;
sinθ = opposite / hypotenuse
Where opposite = h= height, hypotenuse = 13 and θ= 67 degree.
⇒sin67 = h/13
⇒ h = 13sin67
⇒h ≈ 12
Hence, height of traingle ABC = 12
Step 6
Find the base of triangle ABC.
Given that;
BD =AD = 5
⇒ BA = BD + AD = 5 + 5 = 10
Hence, base= 10
Step 7
Calculate the area of triangle ABC .
Using the formula;
Area = base x height
=10 x 12
= 120
Hence, area of triangle ABC = 120
Step 8
Proceed to find the ratio of area of ΔABC to the area of ΔXYZ.
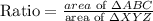
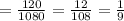
Therefore, the ratio of the area of triangles ABC to the area of triangle XYZ is 1/9