Answer:
a. 15%
b. 60%
Step-by-step explanation:
Part a.
The absolute change in percentage points can be calculated as
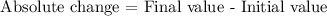
So, by replacing the final value with 40% and the initial value with 25%, we get
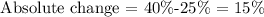
Therefore, the absolute change is 15%
Part b.
The relative change can be calculated as
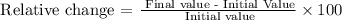
Replacing the final value with 40% and the initial value with 25%, we get

Therefore, the relative change is 60%