Answer:
8.67kJ/mol
Explanations
The formula for calculating the amount of heat absorbed by the water is given as:
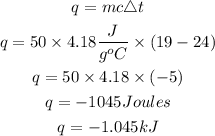
Determine the moles of KI
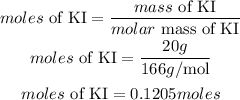
Since heat is lost, hence the enthalpy change of the solution will be negative that is:
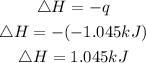
Determine the enthalpy of solution in kJ•mol-1
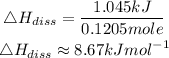
Hence the enthalpy of solution in kJ•mol-1 for KI is 8.67kJ/mol