Answer:
We have the following chemical reaction:
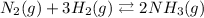
As we can see the equation is balanced because we have the same number of atoms of each element on each side of the equation:
2 nitrogen atoms
6 hydrogen atoms
Now, to answer the question we look at the balanced equation and see that for every mol of N2 that reacts, we need 3 moles of H2 to react. So we calculate the number of moles of H2 needed to react with 0.55 mol of N2 as follows:
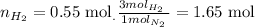
So the answer is 1.65 mol of H2.