The ionic equation is already given, so we can use it as base:
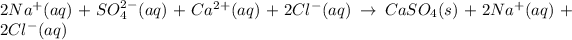
The Net Ionic Equation is the equation for this same reaction but only putting the ions that are actually involved in the reaction. Tha is, we only consider the ions that changed from the left to the right sides.
We can see it as a math equation and if we find any ions that are on both sides, they cancel out.
We can see that we have 2 Na⁺ (aq) ions on both sides, which emans that they are not involved on the reaction and the can be canceled:
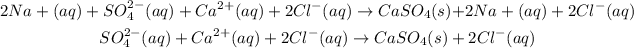
Also, we have 2 Cl⁻ (aq) ions on both sides, so they also are no involved in the reaction, so they can be canceled out:
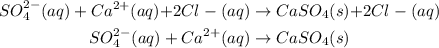
Now, there is no ion appearing on both sides, so all of them are directly involved on the reaction.
This reaction that is left is the Net Ionic Equation, so the answer is:
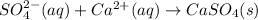
However, there is no alternative for the first option SO₄²⁻. What we can do, is to switch the order of the reactants, because this don't affect the equation.
So, the answer, considering the alternatives, can be written as
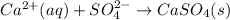
Now, we can pick the fisrt reactant from the alternatives.