Answer
The mole fraction of sulfuric acid in the solution = 0.0939.
Step-by-step explanation
Given that:
The mass of sulfuric acid in the solution = 84 g
The molar mass of sulfuric acid = 98 g/mol
The mass of water in the solution made = 150 g
The molar mass of water = 18 g/mol
What to find:
To find the mole fraction of sulfuric acid in the solution.
Step-by-step solution:
Step 1: Determine the moles of H2SO4 and H2O in the solution.
Using the mole formula, the moles of both the H2SO4 and H2O in the solution can be calculated as follows:
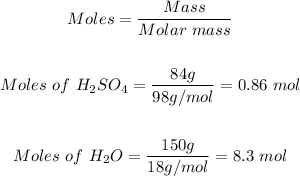
Step 2: Determine the mole fraction of H2SO4 in the solution.
The mole fraction of sulfuric acid in the solution can be calculated using:
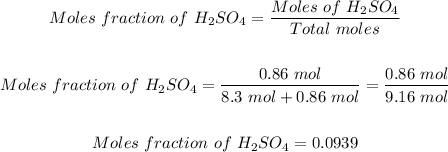
Thus, the mole fraction of sulfuric acid in the solution made is 0.0939.