Answer:
Nitrogen (N2) is the excess reactant
Explanations:
Given the chemical reaction between nitrogen and hydrogen expressed as:
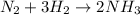
Given the following parameters
Moles of N2 = 2 moles
moles of H2 = 5 moles
Determine the mole of each atom
Mole of one atom of N2 = 2moles/1 = 2moles
Mole of 1 atom of hydrogen = 5moles/3 = 1.67moles
Since moles of one atom of nitrogen is greater then that of hydrogen, hence the excess reactant is Nitrogen