Answer:
d) 464g of NaCl.
e) 424g of the excess reactant.
f) 106g of Na2CO3 remain in excess.
Step-by-step explanation:
d) From part c, we know that 8 moles of NaCl can be produced. To calculate the grams of NaCl we have to convert the 8 moles to grams, using the molar mass of NaCl:
- NaCl molar mass: g/mol
- Conversion:
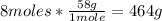
So, 464g of NaCl can be produced.
e) We know from part b that the excess reactant is Na2CO3. From the balanced reaction, we know that 2 moles of HCl react with 1 mole of Na2CO3, so with the 8 moles of the limiting reactant, we can calculate the moles of Na2CO3 that will be needed:
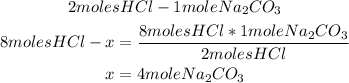
So, only 4 moles of the excess reactant will react. We can calculate the grams using the molar mass of Na2CO3:
- Na2CO3 molar mass: 106g/mol
- Conversion:
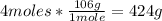
Finally, 424g of the excess reactant react.6
f) To calculate the grams of the excess reactant that remain in excess, it is necessary to convert the 5 moles to grams, using the molar mass of Na2CO3:
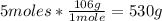
Now we have to subtract the 530g minus the 424g that reacted of Na2CO3:
530g - 424g = 106g
Finally, 106g of Na2CO3 remain in excess.