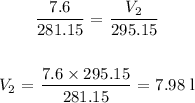Answer:
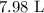
Step-by-step explanation:
Here, we want to get the new volume of the gas without changing the pressure
From Charles' law, we know that temperature and volume are directly proportional
We have that as:
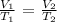
From the question, we have the given values (we have to convert the given temperature values to Kelvin by adding 273.15 K) as:
V1 is 7.6 L
V2 = ?
T1 is 8 + 273.15 = 281.15 K
T2 is 22 + 273.15 = 295.15 K
Substituting the values, we have that as follows: