Answer:
0.74 grams
Explanations
Given the following parameters
Volume of Ca(OH)₂ = 100mL = 0.1L
Molarity of Ca(OH)₂ = 100mM = 0.1M
Determine the moles of Ca(OH)₂
![\begin{gathered} moles\text{ of Ca\lparen OH\rparen}_₂=molarity* volume \\ moles\text{ of Ca\lparen OH\rparen}_₂=(0.1mol)/(L)*0.1L \\ moles\text{ of Ca\lparen OH\rparen^^^^2082=0.01mole} \end{gathered}]()
Calculate the mass of Ca(OH)₂
Molar mass of Ca(OH)2 = 20 + (16*2) + (2*1.01)
Molar mass of Ca(OH)2 = 40 +32 + 2.02
Molar mass of Ca(OH)2 = 74.02g/mol
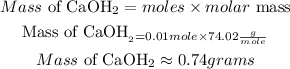
Hence the amount of mass of Ca(OH)2 needed to make 100 ml of a 100mM solution is 0.74 grams