Answer:
The resulting volume is 13.3L.
Step-by-step explanation:
The given information from the exercise is:
- Initial volume (V1): 18.33L
- Initial pressure (P1): 4.648atm
- Final pressure (P2): 6.3998atm
Since the temperature is constant, we can use the Boyle's law formula to calculate the final volume (V2), by replacing the values of V1, T1 and P2:
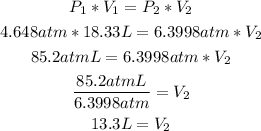
So, the resulting volume is 13.3L.