The neutralization reactions give as products a salt and water.
For the first reaction, the resulting salt will be CaSO4 and for the second NaCl reaction, the reactions obtained are the following:
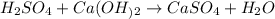

We must count the atoms of each element on both sides of each reaction to check that they are balanced.
For the reaction between H2SO4 and Ca(OH)2 we have:
Reactants side:
H=4atoms
S=1 atoms
O=6atoms
Ca=1 atom
Products side:
H=2atoms
S=1 atoms
O=5atoms
Ca=1 atom
We must balance the hydrogen and oxygen, we place the coefficient two in the water molecule and we will have the equation of the balanced reaction.
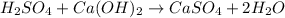
On both sides of the reaction we have:
H=4atoms
S=1 atoms
O=6atoms
Ca=1 atom
For the second reaction, the number of atoms on each side of the reaction is:
H=2atoms
Cl=1atom
Na=1atom
O=1 atom
This equation is already balanced
In summary, we have that the balanced chemical equation for the reactions is:
H2SO4 (aq) + Ca(OH)2 (s) → CaSO4 (s) + 2 H2O (l)
NaOH (aq) + HCl (aq) → NaCl (aq) + H2O (l)