Let's write the chemical equation:
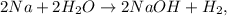
Using the mass of sodium (Na), we can calculate the number of moles of sodium:
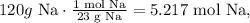
2 mol of Na produces 1 mol of hydrogen gas (2 moles because hydrogen gas contains 2 moles).
We can calculate the number of hydrogen moles required like this:
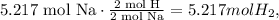
Now, to calculate the mass of hydrogen, we're going to use the molar weight, like this:
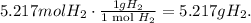
So, hydrogen will be 5.217 g produced by 120 g of sodium.