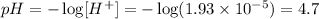We have to remember two things for this kind of problems
1 pH meaning (it is just a formula) ph is negative logarithm of the concentration of protons (H+) in Molar units
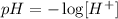
The secong one is that the concentration of protons [H+] times the concentration of inos OH- [OH-] in aquous solution is always 10 to the minus 14:
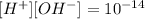
then if they give the concentration of ions OH- we can calcuate the concentration of protons and viceversa:
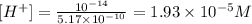
now we have the concentration of protons que only have to apply the pH formula: