The molarity or molar concentration is calculted by dividin the number of moles of solute, n, by the total volume of the solution, V, and normally presented in mol/L.
So:
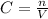
We know that the number of moles of AgNO₃ is 98.1 mol and the volume is 0.250 L, so the molarity, C, will be:
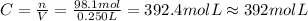
So, the molarity will be approximately 392 mol/L.