Answer
0.7 M
Step-by-step explanation
Given:
The initial volume of the solution, V₁ = 20.0 mL = 0.02 L
The initial molarity of the solution, C₁ = 3.5 M
The final volume of the solution, V₂ = 100.0 mL = 0.10 L
What to find:
The final molarity of the dilute solution, C₂.
Step-by-step solution:
The final molarity of the dilute solution, C₂ can be calculated using the dilution formula below:
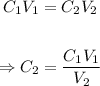
Put the given parameters into the formula:
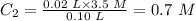
Therefore, the molarity of the dilute solution is 0.7 M