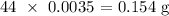Answer:
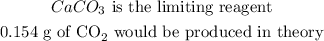
Step-by-step explanation:
We start by completing the balanced equation of the reaction.
We have that as:
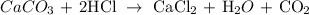
Now, we want to get the limiting reagent
The limiting reagent is the reagent that produces less amount of the product
Now, let us get the number of moles of CaCO3 that reacted
That would be the mass of CaCO3 given, divided by the molar mass of CaCO3
The molar mass of CaCO3 is 100 g/mol
Thus, the number of moles of CaCO3 that reacted will be:
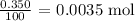
Now, let us get the number of moles of CO2 produced
That would be 0.0035 mol too since the mole ratio of CaCO3 to CO2 in the balanced equation of reaction is 1:1
We proceed to get the number of moles of HCl that reacted
We can get this by multiplying the given molarity by the volume in liters
We have that as:
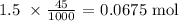
From here, the number of moles of CO2 produced is half, since the mole ratio is 1 to 2
The number of moles of CO2 produced will be:
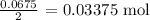
The number of moles of CO2 produced by CaCO3 is lesser, and that means it is the limiting reagent
Now, to get the mass of CO2 produced in theory, we multiply the number of moles by the molar mass of CO2
The molar mass of CO2 is 44 g/mol
Thus, we have the mass that was produced as: