Pb(NO3)2 + 2 NaI ====> 2 NaNO3 + Pbl2
It's already balanced.
We focus only on NaI and Pbl2, so we need their molar mass. (Please use the periodic table to calculate the molar mass)
For NaI = 149.89 g/mol
For Pbl2 = 461.01 g/mol (We use this)
------------------------------------------------------------------------------------------------------------------
We already have 6.0 moles of NaI. So,
2.0 moles of NaI ---------------------- 461.01 g Pbl2
6.0 moles of NaI -------------------- x
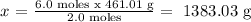
We must write this in scientific notation:
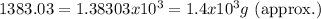
Answer: (A.) 1.4x10^3 g Pbl2