Answer:
232.56M
Explanations:
The formula for calculating the molarity of the solution is expressed as:
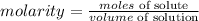
Given the following parameters
moles of solute = 0.05moles
mass of solution (water) = 0.215g
Determine the volume of the solution
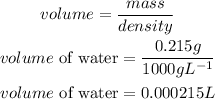
Determine the molarity of the solution

Hence the molarity of a solution that contain 0.050 mol C12H22O11 in 0.215 g water is 232.56M